Data Policy
GAW Data Policy
"For Scientific purposes, access to these data is unlimited and provided without charge.
By their use you accept that an offer of co-authorship will be made through personal contact with the data providers
or owners whenever substantial use is made of their data.
In all cases, an acknowledgement must be made to the data providers or owners and to the data centre when these data are used within a publication."
Version
2025-06-11-0845 (Last updated: 2025-06-11)File
This data set is submitted by NIWA.
In line with the GAW Data Policy, users should contact the contributors of all data of interest and propose co-authorship or acknowledgement.
Organization
| NO | 57 |
|---|---|
| Acronym | NIWA |
| Name | National Institute of Water & Atmospheric Research Ltd. |
| Address 1 | NIWA - Wellington |
| Address 2 | Private Bag 14901, Kilbirnie, Wellington, 6241 |
| Address 3 | 301 Evans Bay Parade, Hataitai, Wellington 6021 |
| Country/Territory | New Zealand |
| Website | http://www.niwa.co.nz |
Contact(s)
| Name | Jocelyn Turnbull |
|---|---|
| Prefix | Dr |
| j.turnbull@gns.cri.nz | |
| Organization No | 120 |
| Organization acronym | GNS |
| Organization name | GNS Science |
| Organization country/territory | New Zealand |
| Address 1 | 30 Gracefield Road |
| Address 2 | Gracefield 5010 |
| Address 3 | New Zealand |
| Country/territory | New Zealand |
| Tel | 045701444 |
| Fax | |
| Last updated date | 2025-07-03 |
| Name | Sylvia Nichol |
|---|---|
| Prefix | |
| sylvia.nichol@niwa.co.nz | |
| Organization No | 57 |
| Organization acronym | NIWA |
| Organization name | National Institute of Water & Atmospheric Research Ltd. |
| Organization country/territory | New Zealand |
| Address 1 | |
| Address 2 | |
| Address 3 | |
| Country/territory | New Zealand |
| Tel | |
| Fax | |
| Last updated date | 2025-07-18 |
| Name | Gordon Brailsford |
|---|---|
| Prefix | |
| gordon.brailsford@niwa.co.nz | |
| Organization No | 57 |
| Organization acronym | NIWA |
| Organization name | National Institute of Water & Atmospheric Research Ltd. |
| Organization country/territory | New Zealand |
| Address 1 | |
| Address 2 | |
| Address 3 | |
| Country/territory | New Zealand |
| Tel | |
| Fax | |
| Last updated date | 2025-08-18 |
| Background observation | |
| UTC+12:00 | |
| permil | |
|
9999-12-31 00:00:00 - 9999-12-31 23:59:59: Delta 14C |
|
|
1954-11-24 00:00:00 - 1995-05-31 23:59:59: Unknown(Decay counting) 1995-06-01 00:00:00 - 2009-04-30 23:59:59: EN Tandem AMS(Mass spectrometry) 1984-11-20 00:00:00 - 9999-12-31 23:59:59: National Electrostatics Corporation AMS(Mass spectrometry) |
|
| fortnightly | |
|
From 1995 to 2010, an EN Tandem AMS was used for measurement. Four graphite targets were prepared from each sample by splitting a single large CO2 aliquot under equilibrium conditions, then reducing to graphite using the LG1 graphite system. Until 2005, only 13C and 14C were measured on the EN Tandem system, so the normalization correction for isotopic fractionation (Stuiver and Polach, 1977) was performed using the IRMS 13C value of the sample measured at NIWA. In 2010, the EN Tandem was replaced with a National Electrostatics Corporation AMS (XCAMS), which measures all three carbon isotopes, such that the normalization correction is performed using the AMS measured 13C values. These measurements are made on single graphite targets, measured to high precision, typically better than 2‰ overall uncertainty. |
|
| The primary source of uncertainty is the 14C counting statistical uncertainty determined from the number of beta decays (gas counting) or 14C counts (AMS). For AMS measurements, we add an additional error term, determined from the long-term repeatability of secondary standard materials and added in quadrature to the AMS uncertainty. In early AMS measurements, this was 4%, and has decreased through time to 0.12%. For the EN Tandem results, this was determined from the performance of OxI primary standard targets through time, which may somewhat overestimate the uncertainty for air samples (Turnbull et al., 2015). For XCAMS measurements, long-term repeatability of air samples has been assessed based on repeated splits of CO2 from a subset of these NaOH samples, as well as repeated extractions of aliquots of CO2 from pressurized tanks of whole air. These repeated aliquots are measured both within the same AMS wheel and across multiple wheels. Within individual wheels, the counting statistical uncertainty is sufficient to explain the full variability, but an additional uncertainty of 0.12% is required to explain the spread across multiple wheels (Turnbull et al., 2015). | |
|
[Hourly] [Daily] [Monthly] |
|
|
A three character flagging system is used. When no flag is indicated, each character is “.”, resulting in “…” for a result with no flags. The first character is a “hard” flag. Any value in this column indicates a sampling or measurement problem and this result should be ignored in any analysis. The second character is a “soft” flag, used to indicate that this result should be excluded from the background record analysis. Here it is used to indicate that the result is an outlier relative to the rest of the record, but there is no positively identified sampling or measurement problem and we therefore cannot remove the datapoint a priori. .T. is used for the period from 1995-2005 when the static NaOH samples have larger scatter than expected, and may be biased high, but are retained in the record in the absence of other data. The third character is informational only and does not indicate a problem with the result. Here we use this flag to indicate changes from the results reported by Manning and Melhuish (1990) and/or Currie et al. (2011) and/or results initially reported from the Rafter AMS database to the NIWA database. ..D is for date of collection and/or decay correction has changed ..N is for normalization correction has changed ..A is for change in how the average of multiple targets from the same sample was calculated. |
|
|
Valid: ... Valid: ..D Valid: ..O Valid: .T. Valid: .TA Valid: .TD Valid: .TN |
|
| Temporarily suspended | |
|
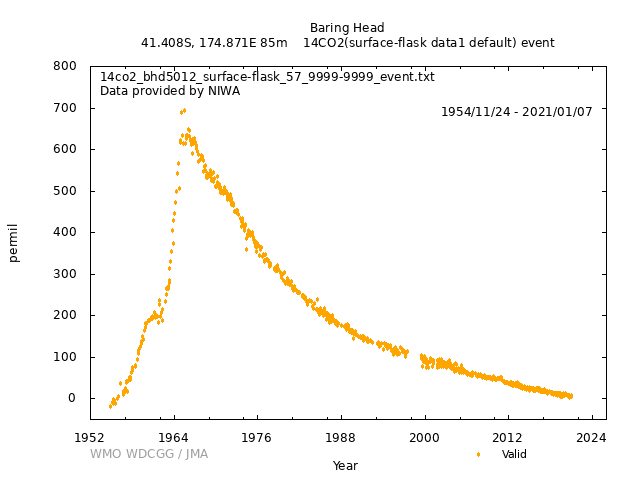
SITE INFORMATION Samples from 1954 - June 1987 were collected at Makara, on the west coast of the North Island of New Zealand (MAK, 41.25°S, 174.69°E, 300 m asl). The sampling site was moved to Baring Head on the South Coast of the North Island and 23 km southeast of Makara (BHD, 41.41°S, 174.87°E, 85 m asl). All samples since 8 July 1988 have been collected at Baring Head. The sampling site can be determined from the Flask_no, where the last letter is "M" for Makara, and "B" for Baring Head. SAMPLE COLLECTION: The primary collection method is static absorption of CO2 into sodium hydroxide (NaOH) solution, which is left exposed to air at the sampling site for ~2 weeks. From 1954-1995, ~ 2 L of the 0.5 M NaOH solution was poured into a large PyrexR tray and left exposed to air for 1 - 2 weeks. Beginning in 1995, when the measurement technique changed from gas counting to AMS measurement, the large trays were replaced with high density polyethylene (HDPE) bottles containing ~200 mL 0.5 M NaOH solution, with the depth of liquid kept the same as it was in the previously used trays. The lid is removed and the bottle is left open inside a Stevenson meteorological screen for ~2 weeks. Date of opening and closing bottle is recorded. The two methods (large tray and small bottle) have been compared, with no significant difference observed between the two methods (Currie et al., 2011). Static NaOH absorption necessarily fractionates relative to CO2 in the atmosphere; typical 13C values are -15 to -25 ‰ for these samples. This is corrected for in the data analysis. Some whole air flask samples from Baring Head have also been analysed for 14C. The collection method can be determined from the Flask_no, where the second to last letter is "T" for tray collection, "B" for bottle collection, and "F" for whole air flask. EXTRACTION METHODS: From 1954- 1995 CO2 was extracted from the NaOH solution by acidification followed by cryogenic distillation (Rafter and Fergusson, 1959). AMS sample extraction follows the general same method, but with smaller volumes (Currie et al., 2011). The 13C is measured at NIWA on an aliquot of CO2 from the same extraction. CO2 is extracted from whole air flask samples by cryogenic extraction. Samples collected from 1984-1993 were extracted and archived as ampoules of pure CO2. In 2012, these tubes were cracked under vacuum to liberate the CO2. Any leakage during storage was readily identified by air present in the tube when it is cracked for transfer and tubes with leakage were discarded. An aliquot of each was measured for 13C to confirm that no fractionation had occurred during storage. Whole air samples collected since 2013 are analyzed for 13C and other trace gases and isotopes at NIWA and for the 14CO2 measurement, CO2 is extracted from whole air at GNS Science. For samples measured using AMS, the LG1 graphitization system was used from 1995 to 2011 and replaced with the RG20 graphite system in 2011. In both systems, CO2 is reduced to graphite over iron catalyst in the presence of hydrogen gas. |
|
|
Wind direction: Wind speed: Relative humidity: Precipitation amount: Air pressure: Air temperature: Dew point temperature: Sea water temperature: Sea surface water temperature: Sea water salinity: Sea surface water salinity: |
|
|
Meteorological data may remain as first provided, even when greenhouse gas data are updated. |
No DOI available
Related information
| Format | Text (WDCGG Data Format Table, WDCGG Meteorological Data Format Table), NetCDF | ||||
|---|---|---|---|---|---|
| Relation List (Is Part Of) |
All 14CO2 data contributed to WDCGG by GAW stations and mobiles by 2025-07-22 All 14CO2 data contributed to WDCGG by GAW stations and mobiles by 2022-07-11 All 14CO2 data contributed to WDCGG by GAW stations and mobiles by 2020-12-18 All 14CO2 data contributed to WDCGG by GAW stations and mobiles by 2019-09-03 All 14CO2 data contributed to WDCGG by GAW stations and mobiles by 2018-06-26 |
||||
| Geolocation Point |
|
GAW Data Policy
"For Scientific purposes, access to these data is unlimited and provided without charge.
By their use you accept that an offer of co-authorship will be made through personal contact with the data providers
or owners whenever substantial use is made of their data.
In all cases, an acknowledgement must be made to the data providers or owners and to the data centre when these data are used within a publication."
Citation format
This format is an example of the WDCGG standard citation.
Please follow the citation format which the data providers or owners indicate.
Please follow the citation format which the data providers or owners indicate.
Jocelyn Turnbull (GNS),
Sylvia Nichol (NIWA),
Gordon Brailsford (NIWA),
Atmospheric 14CO2
at Baring Head by National Institute of Water & Atmospheric Research Ltd.,
dataset published as 14CO2_BHD5012_surface-flask_NIWA_data1 at WDCGG,
ver. 2025-06-11-0845 (Reference date*: YYYY/MM/DD)
* As the reference date, please indicate the date you downloaded the files.
* As the reference date, please indicate the date you downloaded the files.
Reference(s)
| 1 | Turnbull, J. C., Mikaloff Fletcher, S. E., Ansell, I., Brailsford, G., Moss, R., Norris, M., and Steinkamp, K.: Sixty years of radiocarbon dioxide measurements at Wellington, New Zealand 1954-2014, Atmos. Chem. Phys. Discuss., doi:10.5194/acp-2016-1110, in review, 2016. |
|---|---|
| 2 | Currie, Kim I, Gordon Brailsford, Sylvia Nichol, Antony Gomez, Rodger Sparks, Keith R Lassey, Katja Reidel, (2011) Tropospheric 14CO2 at Wellington, New Zealand - the world’s longest record. Biogeochemistry 104:5-22 DOI 10.1007/s10533-009-9352-6. |
| 3 |
Manning MR, Lowe DC, Melhuish WH, Sparks RJ, Wallace G, Breninkmeijer CAM, McGill RC (1990) The use of radiocarbon measurements in atmospheric studies. Radiocarbon 32: 37-58 |
| 4 |
Rafter TA, Fergusson GJ Atmospheric radiocarbon as a tracer in geophysical circulation problems. In: United Nations Peaceful Uses of Atomic Energy. Pergamon Press, London. 1959. Pergamon Press |
| 5 | Stuiver M, Polach HA (1977) Reporting of 14C data. Radiocarbon 19(3): 355 - 363 |